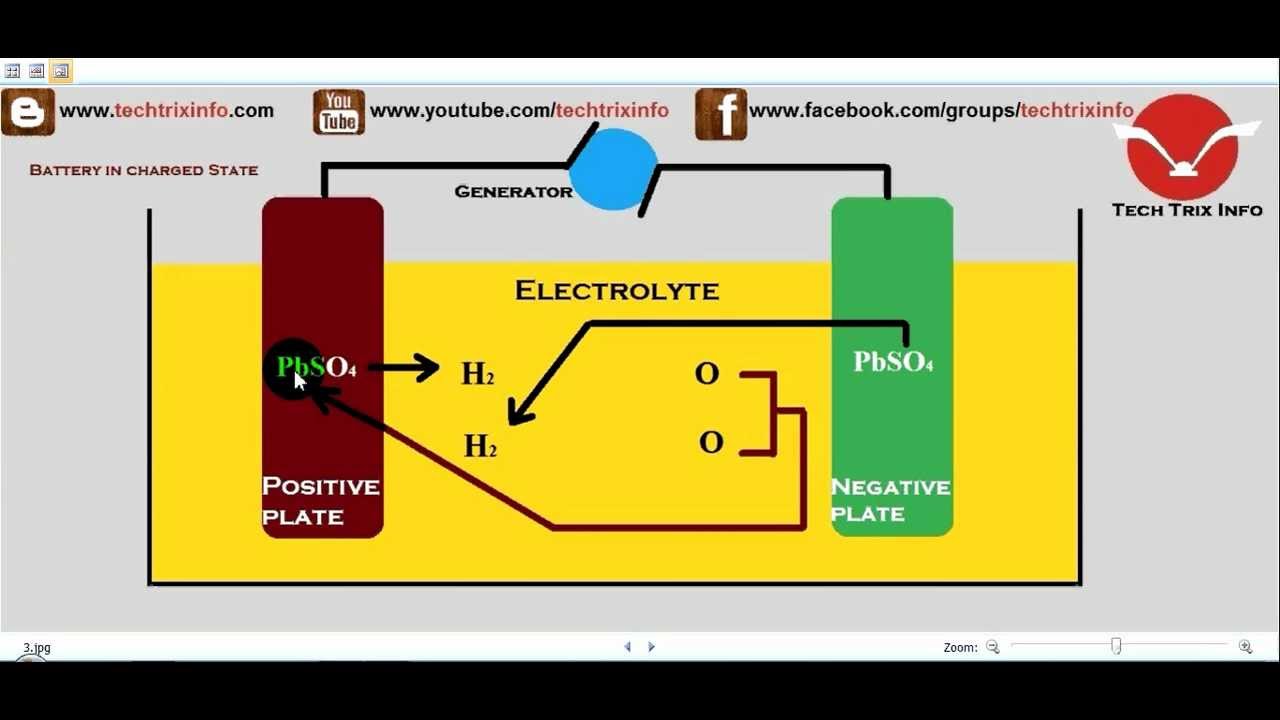
Lead Acid Battery Equation . the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate the formation and dissolution of lead. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. a lead acid battery consists of a negative electrode made of spongy or porous lead.
from www.youtube.com
the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead is porous to facilitate the formation and dissolution of lead. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. a lead acid battery consists of a negative electrode made of spongy or porous lead.
How lead acid battery works. YouTube
Lead Acid Battery Equation a lead acid battery consists of a negative electrode made of spongy or porous lead. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate the formation and dissolution of lead. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. a lead acid battery consists of a negative electrode made of spongy or porous lead. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example.
From orvelleblog.blogspot.com
Spice of Lyfe Lead Acid Battery Chemical Reaction Equation Lead Acid Battery Equation lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. a lead acid battery consists of a negative electrode made of spongy or porous lead. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. The. Lead Acid Battery Equation.
From www.youtube.com
Lead Acid Battery How Car Battery Works? Automobile Battery Working Lead Acid Battery Equation lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead is porous to facilitate the formation and dissolution of lead. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. a lead acid battery. Lead Acid Battery Equation.
From www.youtube.com
Explanation to lead acid battery working principle in detail. YouTube Lead Acid Battery Equation a lead acid battery consists of a negative electrode made of spongy or porous lead. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead is porous to facilitate the formation and dissolution of lead. The reaction of lead and lead oxide with. Lead Acid Battery Equation.
From studylib.net
1. LeadAcid Battery 1 Wayne State University Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. . Lead Acid Battery Equation.
From www.slideserve.com
PPT Commercial Voltaic Cells PowerPoint Presentation, free download Lead Acid Battery Equation explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate. Lead Acid Battery Equation.
From www.chegg.com
Solved A 12V leadacid battery characteristics can be Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate the formation and dissolution of lead. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. a lead acid battery consists of a negative. Lead Acid Battery Equation.
From electricalacademia.com
What is a LeadAcid Battery? Construction, Operation, and Charging Lead Acid Battery Equation explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. a. Lead Acid Battery Equation.
From engineeringhulk.com
Lead Acid Battery Construction, Working, Advantages Lead Acid Battery Equation explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte. Lead Acid Battery Equation.
From www.electroniclinic.com
Lead acid battery, Construction and, Working, and Charging Lead Acid Battery Equation The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. The lead is porous to facilitate the formation and dissolution of lead. a lead acid battery consists of a negative electrode made of spongy or. Lead Acid Battery Equation.
From www.electricity-magnetism.org
Composition of Leadacid Battery Anode, Cathode & Electrolyte Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. explore the process of balancing redox reactions in acidic solutions using a lead storage battery as an example. a lead acid battery consists. Lead Acid Battery Equation.
From www.nagwa.com
Question Video Identifying the Reaction That Occurs at the Positive Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead is porous to facilitate the formation and dissolution of lead. The reaction of lead. Lead Acid Battery Equation.
From www.researchgate.net
Standard leadacid battery Download Scientific Diagram Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate the formation and dissolution of lead. a lead acid battery consists of a negative electrode made of spongy or porous lead. The reaction of lead and lead oxide with the sulfuric acid electrolyte. Lead Acid Battery Equation.
From www.slideserve.com
PPT The Lead Acid Electric Battery PowerPoint Presentation, free Lead Acid Battery Equation The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead is porous to facilitate the formation and dissolution of lead. a lead acid battery consists of a negative electrode made of spongy or porous lead. lead and lead dioxide, the active materials on the plate of the battery, react to lead. Lead Acid Battery Equation.
From www.youtube.com
Working Principle of Lead Acid Battery YouTube Lead Acid Battery Equation the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead is porous to facilitate the formation and dissolution of lead. explore the process. Lead Acid Battery Equation.
From instrumentationtools.com
Discharge and Charging of LeadAcid Battery Inst Tools Lead Acid Battery Equation a lead acid battery consists of a negative electrode made of spongy or porous lead. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead is porous to facilitate the formation and dissolution of lead. The reaction of lead and lead oxide with the sulfuric acid electrolyte. Lead Acid Battery Equation.
From www.researchgate.net
Leadacid battery construction. Download Scientific Diagram Lead Acid Battery Equation lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. . Lead Acid Battery Equation.
From www.youtube.com
How a lead acid battery works YouTube Lead Acid Battery Equation The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. a lead acid battery consists of a negative electrode made of spongy or porous lead. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. explore the process of balancing redox reactions in. Lead Acid Battery Equation.
From www.junleepower.com
Leadacid Battery Discharge CurveEquation Lead Acid Battery Equation The lead is porous to facilitate the formation and dissolution of lead. lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. the lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. a lead acid. Lead Acid Battery Equation.